
In the language of statistical mechanics, entropy is a measure of the number of microscopic configurations corresponding to a macroscopic state. The second law is an empirically validated postulate of thermodynamics, but it can be understood and explained using the underlying quantum statistical mechanics, together with the assumption of low-entropy initial conditions in the distant past (possibly at the beginning of the universe).
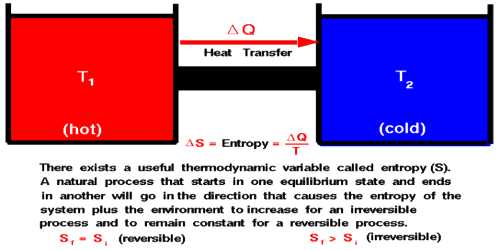
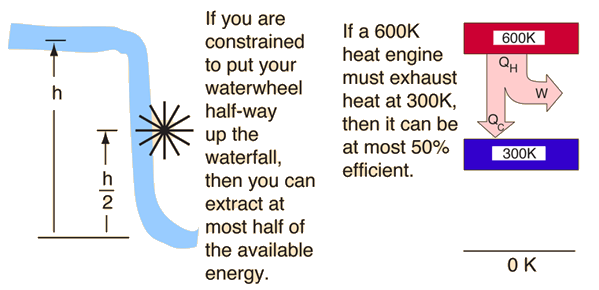
Equivalently, perpetual motion machines of the second kind are impossible. The second law of thermodynamics states that the entropy of an isolated system never decreases because isolated systems spontaneously evolve towards thermodynamic equilibrium-the state of maximum entropy.

To Download Second Law Of Thermodynamics Click Here


 0 kommentar(er)
0 kommentar(er)
